-
What are some of the major types of lead acid batteries?
Batteries are divided in two ways, by application (what they are used for) and construction (how they are built). The major applications are automotive, marine, and deep-cycle. Deep-cycle includes solar electric (PV), backup power, and RV and boat “house” batteries. The major construction types are flooded (wet), gelled, and AGM (Absorbed Glass Mat). AGM batteries are also sometimes called “starved electrolyte” or “dry”, because the fiberglass mat is only 95% saturated with Sulfuric acid and there is no excess liquid. Flooded may be standard, with removable caps, or the so-called “maintenance free” (that means they are designed to die one week after the warranty runs out). All gelled are sealed and a few are “valve regulated”, which means that a tiny valve keeps a slight positive pressure. Nearly all AGM batteries are sealed valve regulated (commonly referred to as “VRLA” – Valve Regulated Lead-Acid). Most valve-regulated are under some pressure – 1 to 4 psi at sea level.
-
Where do I recycle my old batteries?
-
Are lead acid batteries recyclable?
Lead acid batteries are 100% recyclable. Lead is the most recycled metal in the world today. The plastic containers and covers of old batteries are neutralized, reground and used in the manufacture of new battery cases. The electrolyte can be processed for recycled waste water uses. In some cases, the electrolyte is cleaned and reprocessed and sold as battery grade electrolyte. In other instances, the sulfate content is removed as Ammonia Sulfate and used in fertilizers. The separators are often used as a fuel source for the recycling process.
-
Is there a maximum temperature for charging lead acid batteries?
When charging lead acid batteries, the temperature should not exceed 120°F. At this point the battery should be taken off charge and allowed to cool before resuming the charge process.
-
Can batteries freeze?
In a partially discharged state, the electrolyte in a lead acid battery may freeze. At a 40% state of charge, electrolyte will freeze if the temperature reaches approximately ¬16.0°F. The freezing temperature of the electrolyte in a fully charged battery is -92.0°F.
-
What is the proper electrolyte level?
Liquid levels should be 1/8 inch below the bottom of the vent well (the plastic tube that extends into the battery). The electrolyte level should not drop below the top of the plates.
-
What is Electrolyte?
In a lead-acid battery, the electrolyte is sulfuric acid diluted with water. It is a conductor that supplies water and sulfate for the electrochemical reaction.
-
What is OHM’S Law?
OHM’S Law expresses the relationship between volts (V) and amperes (A) in an electrical circuit with resistance (R). It can be expressed as follows: V= IR Volts (V) = Amperes (I) x Ohms (R). If any two of the three values are known, the third value can be calculated using the above equation.
-
What is an OHM?
OHM is a unit for measuring electrical resistance or impedance within an electrical circuit.
-
What is a WATT?
A WATT is the unit for measuring electrical power, i.e., the rate of doing work, in moving electrons by, or against, an electrical potential. Formula: Watts = Amperes x Volts.
-
What is a VOLT?
A Volt is the unit of measure for electrical potential.
-
What is a Battery?
A battery can be any device that stores energy for later use. The word battery is limited to an electrochemical device that converts chemical energy into electricity, by use of a galvanic cell. A galvanic cell is a fairly simple device consisting of two electrodes (an anode and a cathode) and an electrolyte solution. Batteries consist of one or more galvanic cells.
A battery is an electrical storage device. Batteries do not make electricity, they store it. As chemicals in the battery change, electrical energy is stored or released. In rechargeable batteries this process can be repeated many times. Batteries are not 100% efficient – some energy is lost as heat and chemical reactions when charging and discharging. If you use 1000 watts from a battery, it might take 1200 watts or more to fully recharge it. Slower charging and discharging rates are more efficient. A battery rated at 180 amp-hours over 6 hours might be rated at 220 AH at the 20-hour rate and 260 AH at the 48-hour rate. Typical efficiency in a lead-acid battery is 85-95%, in alkaline and NiCad battery it is about 65%.
-
What is sulfation of batteries?
Sulfation is the formation or deposit of lead sulfate on the surface and in the pores of the active material of the batteries’ lead plates. If the sulfation becomes excessive and forms large crystals on the plates, the battery will not operate efficiently and may not work at all. Common causes of battery sulfation are standing a long time in a discharged condition, operating at excessive temperatures, and prolonged under or over charging.
-
What considerations should I use when deciding between sealed batteries vs. flooded batteries?
The sealed batteries (VRLA- valve regulated lead acid) have come a long way since their entrance into the backup power industry in the late 70’s. Many have used this battery successfully for years. The advantages over the flooded battery being; the minimal amount of maintenance required, the smaller footprint for the same capacity, the minimal amount of gassing under normal conditions, ease of installation and an easing of requirements for spill containment and venting.
The flooded battery on the other hand has been around for over a hundred years and many feel that it is more reliable and worth the added expense involved in freight, installation, maintenance, etc. Application and personal preference play a big part in this decision.
-
How are batteries rated and what do the ratings mean in battery selection?
The most common battery rating is the AMP-HOUR RATING. This is a unit of measurement for battery capacity, obtained by multiplying a current flow in amperes by the time in hours of discharge. (Example: a battery which delivers 25 amperes for 8 hours delivers 25 amperes times 8 hours, or 200 ampere-hours.)
Manufacturers use different discharge periods to yield different Amp-Hr. Rating for the same capacity batteries, therefore, the Amp-Hr. Rating has little significance unless qualified by the number of hours the battery is discharged. For this reason Amp-Hour Ratings are only a general method of evaluating a battery’s capacity for selection purposes. The quality of internal components and technical construction within the battery will generate different desired characteristics without effecting its Amp-Hour Rating. For instance, there are 150 Amp-Hour batteries that will not support an electrical load overnight and if called upon to do so repetitively, will fail early in their life. Conversely, there are 150 Amp-Hour batteries that will operate an electrical load for several days before needing recharging and will do so for years.
-
What are the minimum battery requirements to maintain my warranty?
This varies by manufacturer. At a minimum, the user is required to annually measure and record voltage and temperature readings. Some manufacturers also require specific gravity readings (flooded battery) and cyclic readings (how many times the battery is discharged and recharged).
-
How can I evaluate the health and charge state of a battery?
Routine battery examinations divulge irregularities in the charging system as well as in the batteries. The principle method is to examine the electrochemistry of the battery through hydrometric electrolyte inspection. Please keep in mind that this important examination cannot be accomplished with sealed absorption or gel batteries. Voltage readings alone require experience to interpret. Hydrometric readings will uncover early warnings of over-charging or over-discharging before batteries are damaged. The state-of-charge and reliability of a lead acid battery can best be determined by the specific gravity of the electrolyte measured directly with a common bulb-type hydrometer with a glass float. Specific gravity is a unit of measurement for determining the sulfuric acid content of the electrolyte. The recommended fully charged specific gravity of lead-acid, flooded batteries is 1.260 taken at 77°F. More than .025 spread in readings between fully charged cells indicates that the battery may need an equalization charge. If this condition persists, the cell is failing and the battery should be replaced. Since water has a value of 1.000, electrolyte with a specific gravity of 1.260 means it is 1.260 times heavier than pure water while pure concentrated sulfuric acid has a specific gravity of 1.835.
The following table illustrates typical specific gravity values for a cell in various stages of charge:
100% Charged…….1.255 – 1.260 Sp. Gr.
75% Charged…….1.220 – 1.225 Sp. Gr.
50% Charged…….1.185 – 1.190 Sp. Gr.
25% Charged…….1.150 – 1.155 Sp. Gr.
0% Charged…….1.115 – 1.120 Sp. Gr.
Temperature compensation of hydrometric readings is usually unnecessary unless the battery is extremely hot or cold, however, after hard charging or discharging, you may want to add or subtract points of Specific Gravity based on the table. Also, hydrometer readings taken immediately after water is added to a cell is inaccurate. The water must be thoroughly mixed with the underlying electrolyte by charging, before hydrometer readings are reliable. In addition, do not assume a deep cycle battery will not take a charge because you have been charging it for a while and the float will not rise. If the battery has been fully discharged or partially sulfated it will require considerable charging or equalization before recovering. As electrolyte levels are reduced in the battery, it is important to add water to each cell. Note that only the water portion of the electrolyte evaporates, therefore, it is not necessary to add acid to a battery during maintenance. In fact, the addition of acid to an active battery will reduce its capacity and shorten its remaining life. Water should be added to cells after charging the battery. This will eliminate spillage due to expansion of electrolyte upon charging. Generally speaking, any water that is safe to drink is safe to use in a battery. Do not use water of a known high mineral content or stored in metallic containers. It is the metal impurities in the water that lower the performance of the battery. Distilled water guarantees purity.
-
What kind of battery testing should be performed to insure that I have enough battery backup?
There are a number of good testing methods available, i.e., resistance, impedance, and conductance which will provide good indicators of the strength of one battery cell compared to the next or how your battery today compares with itself when it was new (as long as initial readings were taken). These tests however do not provide a true indication of the capacity of the battery.
The best and only way to truly test battery capacity is to apply a load test by either using the load of the equipment being backed up or by using a resistive load bank. This test when performed per IEEE specifications will provide the owner with the facts required to determine whether or not a battery needs to be replaced.
-
Will the battery be adversely affected if stored at warmer temperatures?
Yes. For every 18 degrees F above 77 degrees, the time interval for the initial charge and recharge should be halved. If a battery was stored at 95 degrees F the maximum storage interval would be 3 months. Storage beyond these periods without proper charge can result in excessive sulfating of plates and positive grid corrosion which is detrimental to battery performance and life.
-
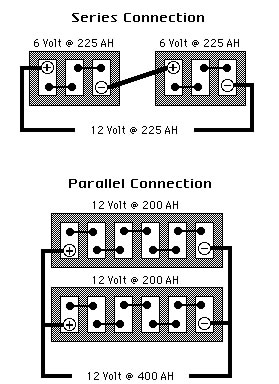
What is the difference between series battery connections and parallel battery connections and how do they increase battery capacity and voltage?
In the SERIES CONNECTION, batteries of like voltage and Amp-Hour capacity are connected to increase the Voltage of the bank. The positive terminal of the first battery is connected to the negative terminal of the second battery and so on, until the desired voltage is reached. The final Voltage is the sum of all battery voltages added together while the final Amp-Hours remains unchanged. The bank’s Voltage increases while its Amp-Hours, Cranking Performance and Reserve Capacity remain unchanged.
In the PARALLEL CONNECTION, batteries of like voltages and capacities are connected to increase the capacity of the bank. The positive terminals of all batteries are connected together, or to a common conductor, and all negative terminals are connected in the same manner. The final voltage remains unchanged while the capacity of the bank is the sum of the capacities of the individual batteries of this connection. Amp-Hours Cranking Performance and Reserve Capacity increases while Voltage does not.
-
What is the storage life for stationary batteries?
The storage interval from date of shipment to date of installation and initial charge should not exceed six (6) months. If stored at temperatures 77 degrees F or below, the battery should be given its initial charge at or before 6 months and recharged at 6 month intervals.
Comments are closed.